Photocatalyzed cascade oxidative annulation of propargylamines and phosphine oxides†
Abstract
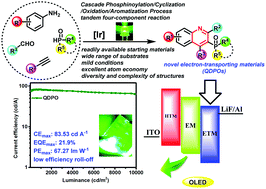
On account of the broad utilities of organophosphorus compounds, the development of highly efficient and concise phosphination methods is significantly important and urgent. Herein, we disclose a novel method for the synthesis of phosphorylated heterocycles: versatile intermediate propargylamines serving as a new type of radical acceptors incorporated in P-radicals via a photocatalytic strategy. This reaction proceeds through a cascade phosphinoylation/cyclization/oxidation/aromatization pathway using readily available starting materials under mild conditions of light with excellent atom economy, catalyzed by AgOAc or fac-Ir(ppy)3. One of the phosphorylated quinolines was selected, as an example, as an electron-transporting material for fabricating phosphorescence organic light-emitting diodes displaying excellent electroluminescence performances with a maximum external quantum efficiency of 21.9% with negligible efficiency roll-off ratios.


 Please wait while we load your content...
Please wait while we load your content...