Crystallographic analyses of isoquinoline complexes reveal a new mode of metallo-β-lactamase inhibition†
Abstract
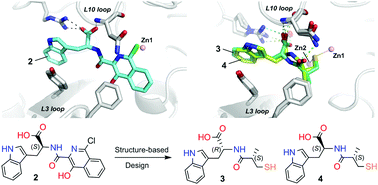
Crystallographic analyses of the VIM-5 metallo-β-lactamase (MBL) with isoquinoline inhibitors reveal non zinc ion binding modes. Comparison with other MBL–inhibitor structures directed addition of a zinc-binding thiol enabling identification of potent B1 MBL inhibitors. The inhibitors potentiate meropenem activity against clinical isolates harboring MBLs.


 Please wait while we load your content...
Please wait while we load your content...