Exploiting the unusual effects of fluorine in methodology
Abstract
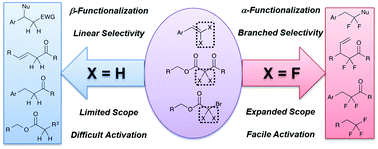
Fluorination of organic molecules significantly impacts the basic physicochemical properties of small and large biologically active molecules, agrichemicals, and materials. Thus, the development of synthetic reactions to access these substructures is important for many applied fields of chemistry. However, these fluorine-induced perturbations of chemical properties can inhibit standard chemical transformations, which provides unique challenges for synthetic organic chemists. In addition, the physicochemical properties imparted by fluorinated substituents can enable distinct reactivity patterns relative to non-fluorinated substrates, thus making synthetic organofluorine chemistry a fertile ground for developing new, exciting transformations. In this feature article, we detail our experiences in methodology, wherein fluorinated substrates have enabled unique reactivity patterns relative to non-fluorous substrates. Specifically, we highlight the non-standard chemo- and regio-selectivities imparted by fluorinated substrates on Pd-catalyzed coupling reactions, nucleophilic addition reactions of olefins, and Cu-catalyzed decarboxylative fluoroalkylation reactions.
- This article is part of the themed collection: 2017 Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...