Pinpointing disulfide connectivities in cysteine-rich proteins†
Abstract
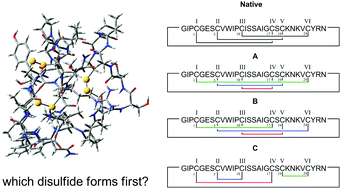
A simple MD-based protocol is presented to accurately predict both the sequence and order of disulfide bond formation in proteins containing multiple cysteine residues. It provides a detailed description of their dynamical and structural features, which can be used to perform ensemble-averaged ECD calculations. Plant cyclotides are used as model compounds.
- This article is part of the themed collection: 2017 Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...