Efficient synthesis of cyclic P-stereogenic phosphinamides from acyclic chiral precursors via radical oxidative intramolecular aryl C–H phosphinamidation†
Abstract
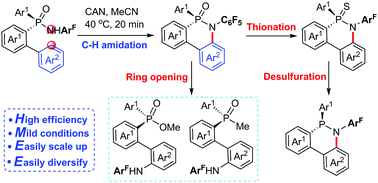
We present a highly efficient method for the synthesis of cyclic P-stereogenic phosphinamides via the Ce(IV)-promoted radical oxidative aryl C–H phosphinamidation of acyclic P-stereogenic phosphinamides. The new protocol provides a useful platform for the versatile synthesis of various potentially useful P-stereogenic compounds.


 Please wait while we load your content...
Please wait while we load your content...