Alkynyl sulfoxides as α-sulfinyl carbene equivalents: gold-catalysed oxidative cyclopropanation†
Abstract
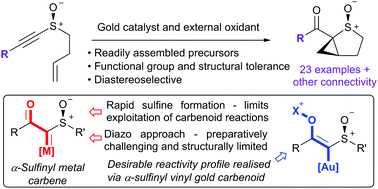
Alkynyl sulfoxides are shown to act as α-sulfinyl metallocarbene synthons under oxidative gold catalysis, enabling reactions that are not available from diazo-precursors. This strategy is exemplified in the synthesis of fused α-sulfinyl cyclopropanes.


 Please wait while we load your content...
Please wait while we load your content...