Distinguishing between homogeneous and heterogeneous hydrogen-evolution catalysis with molecular cobalt complexes†
Abstract
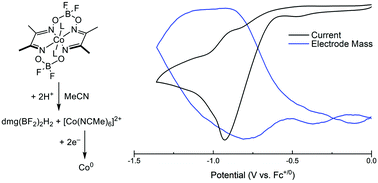
Molecular proton-reduction catalysts can decompose under the acidic conditions required for hydrogen evolution, resulting in formation of secondary metal-containing materials. Using an electrochemical quartz crystal microbalance (EQCM), we report a new method for probing electrodeposition of catalytically active heterogeneous material from molecular precursors. The data collected suggest that EQCM can provide a direct measure of the homogeneity of molecular proton-reduction catalysts over short timescales.
- This article is part of the themed collection: 2017 Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...