A dynamic kinetic asymmetric transfer hydrogenation–cyclization tandem reaction: an easy access to chiral 3,4-dihydro-2H-pyran-carbonitriles†
Abstract
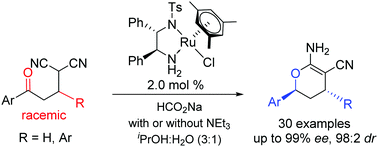
A chiral (mesitylene)RuCl(monosulfonated diamine) catalysed dynamic kinetic resolution (DKR)–asymmetric transfer hydrogenation (ATH) process is developed for highly enantio- (up to 99% ee) and diastereo- (up to 98 : 2 dr) selective reduction of challenging racemic α-aryl-γ-keto malononitriles. A spontaneous cyclization reaction of the hydrogenation products delivers a cascade process for efficient synthesis of useful enantioenriched 3,4-dihydro-2H-pyran-carbonitriles.


 Please wait while we load your content...
Please wait while we load your content...