Ag-Catalyzed difluorohydration of β-alkynyl ketones for diastereoselective synthesis of 1,5-dicarbonyl compounds†
Abstract
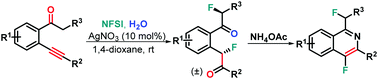
A new catalytic difluorohydration of β-alkynyl ketones using NFSI as the fluorinating reagent has been established, diastereoselectively furnishing a range of structurally diverse difluoride 1,5-dicarbonyl products through C(sp3)–H fluorination. Notably, the sterically encumbered t-butyl functionality located at the α-position of the carbonyl group of substrates 1 showed excellent diastereoselectivity (up to >99 : 1 dr). The reaction enabled multiple bond-forming events including two C(sp3)–F formation through Ag-catalysis to provide a highly efficient and practical method toward difluoride 1,5-dicarbonyls, some of which were successfully converted into difluorinated isoquinolines.


 Please wait while we load your content...
Please wait while we load your content...