Access to divergent benzo-heterocycles via a catalyst-dependent strategy in the controllable cyclization of o-alkynyl-N-methoxyl-benzamides†
Abstract
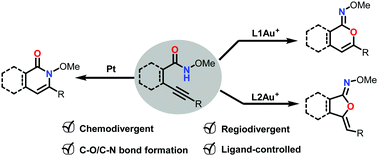
A chemo- and regio-selectively controllable approach for construction of diverse benzo-heterocycles is established. A new strategy for using the ligand effect in gold catalysis to control the regioselectivity in the cyclization of o-alkynyl-N-methoxyl-benzamide is successfully achieved. Meanwhile, the chemoselectivity between nitrogen and oxygen nucleophiles is precisely switched by gold and platinum catalysts.


 Please wait while we load your content...
Please wait while we load your content...