Development of an enantioselective amine–silver co-catalyzed Conia-ene reaction†
Abstract
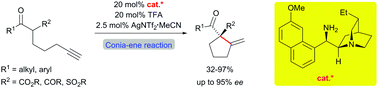
The development of a novel cooperative catalytic system for an amine–silver co-catalyzed Conia-ene reaction of alkyne-tethered C–H-acidic compounds is reported. By using a cost-effective silver salt and a small diamine for the 5-exo-dig-cyclization the cyclopentane products are obtained in very good yields. The enantioselectivity of the reaction could be controlled by exchanging the diamine co-catalyst with a cinchona-derived primary amine.


 Please wait while we load your content...
Please wait while we load your content...