An asymmetric vinylogous Mukaiyama–Michael reaction of α,β-unsaturated 2-acyl imidazoles catalyzed by chiral Sc(iii)– or Er(iii)–pybox complexes†
Abstract
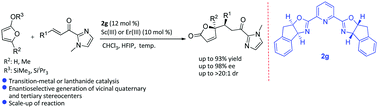
A highly diastereo- and enantioselective vinylogous Mukaiyama–Michael reaction of silyloxyfurans with α,β-unsaturated 2-acyl imidazoles catalyzed by either chiral Sc(III) or Er(III) complexes of a pybox ligand has been reported. The enantioenriched γ-butenolides formed in the reaction were further transformed into highly functionalized γ-lactones found as important structural frameworks in a wide range of biologically active natural products.


 Please wait while we load your content...
Please wait while we load your content...