Diversification reactions of γ-silyl allenyl esters: selective conversion to all-carbon quaternary centers and γ-allene dicarbinols†
Abstract
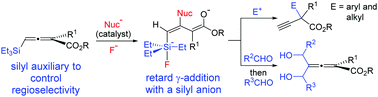
The unique reactivity of γ-silyl allenyl esters is described. Taking advantage of the silyl group as a fluoride acceptor, these allenoates readily underwent addition to a variety of electrophiles to selectively yield products with all-carbon quaternary centers or allenoate dicarbinols. These dicarbinols were subsequently converted to novel highly substituted 6-hydro-2-pyrones.


 Please wait while we load your content...
Please wait while we load your content...