Dual-role of PtCl2 catalysis in the intramolecular cyclization of (hetero)aryl-allenes for the facile construction of substituted 2,3-dihydropyrroles and polyheterocyclic skeletons†
Abstract
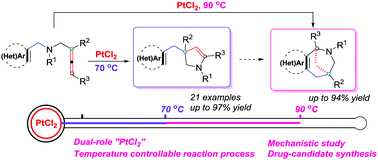
A Pt(II)-catalyzed cyclization of (hetero)aryl-allenes has been developed, providing controllable synthesis of substituted 2,3-dihydropyrroles and polyheterocyclic skeletons. Another notable feature of this method is the dual-role of the Pt(II) catalyst: initiation of the migration of the (hetero)arylmethylene group and the subsequent Friedel–Crafts type annulation.


 Please wait while we load your content...
Please wait while we load your content...