Integrating natural biomass electro-oxidation and hydrogen evolution: using a porous Fe-doped CoP nanosheet array as a bifunctional catalyst†
Abstract
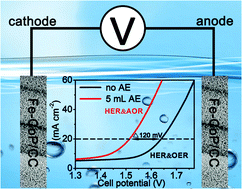
Electrochemical water splitting has been considered as a promising strategy for hydrogen production, but the sluggish anodic oxygen evolution reaction (OER) limits the efficiency of overall water splitting. In this communication, we report a facile strategy to realize energy-saving hydrogen generation by replacing OER with a thermodynamically more favorable aloe extract (AE) oxidation reaction (AOR). An Fe-doped CoP nanosheet array (Fe-CoP/CC) is used as a bifunctional catalyst for both AOR and hydrogen evolution reaction (HER). The Fe-CoP/CC∥Fe-CoP/CC couple requires a cell voltage of 1.51 V to drive 20 mA cm−2 in 1.0 M KOH containing AE; however, a cell volatge of 1.63 V is required to drive the same current density in the absence of AE. Fe-CoP/CC also exhibits strong long-term electrochemical durability and nearly 100% faradaic efficiency.
- This article is part of the themed collection: Most cited articles from 2017


 Please wait while we load your content...
Please wait while we load your content...