Borinic acid-catalyzed stereo- and site-selective synthesis of β-glycosylceramides†
Abstract
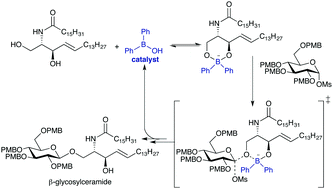
A method for activation of unprotected ceramides towards stereo- and site-selective glycosylation is described. Two-point binding of a diarylborinic acid catalyst to the ceramide accelerates its reactions with ‘armed’ glycosyl methanesulfonate donors, resulting in the formation of a β-glycosidic linkage at the primary OH group.
- This article is part of the themed collection: Site-selective molecular transformations


 Please wait while we load your content...
Please wait while we load your content...