Polyfluoroarylation of oxazolones: access to non-natural fluorinated amino acids†
Abstract
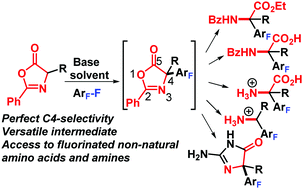
Herein, conditions are provided for the formation and use of the oxazolone enolate for the nucleophilic substitution of highly fluorinated (hetero)arenes, which after unmasking yield highly fluorinated non-natural amino acids and derivatives. In addition, the properties and chemical behavior of this new class of amino acids are explored. The utility is demonstrated in the one pot synthesis of medicinally relevant 2-aminohydantoins.


 Please wait while we load your content...
Please wait while we load your content...