Metallic reductant-free synthesis of α-substituted propionic acid derivatives through hydrocarboxylation of alkenes with a formate salt†
Abstract
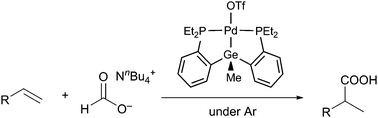
A PGeP–pincer palladium-catalyzed hydrocarboxylation of styrenes to obtain pharmaceutically important α-arylpropionic acid derivatives was achieved using a formate salt as both a reductant and a CO2 source. The reaction was also applicable to vinylsulfone and acrylates. Isotope labeling experiments demonstrated that a CO2-recycling mechanism is operative through generation and reaction of a benzylpalladium complex as a carbon nucleophile. This protocol has realized a mild and atom economical CO2-fixation reaction without the necessity of using strong metallic reductants.


 Please wait while we load your content...
Please wait while we load your content...