Targeting secondary protein complexes in drug discovery: studying the druggability and chemical biology of the HSP70/BAG1 complex†
Abstract
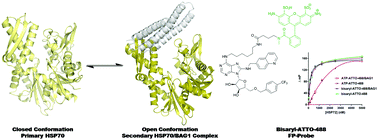
Proteins typically carry out their biological functions as multi-protein complexes, which can significantly affect the affinity of small-molecule inhibitors. HSP70 is an important target in oncology, so to study its chemical biology and the drug discovery potential of the HSP70/BAG1 complex, we designed a high-affinity non-nucleotide fluorescence polarisation probe.


 Please wait while we load your content...
Please wait while we load your content...