N-Difluoromethyl-triazole as a constrained scaffold in peptidomimetics†
Abstract
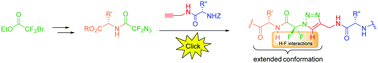
The N-difluoromethyl triazolo-β-aza-ε-amino acid present in the core of peptides led to constrained conformations due to CH–F and NH–F interactions. Pseudotetrapeptides were obtained in excellent yields directly by click chemistry between azidodifluoroacetamides and alkynes, both linked to an amino acid. This work demonstrates that the N-difluoromethyltriazole scaffold can induce extended structures to β-strand mimics.


 Please wait while we load your content...
Please wait while we load your content...