Development of emissive aminopentaazaphenalene derivatives employing a design strategy for obtaining luminescent conjugated molecules by modulating the symmetry of molecular orbitals with substituent effects†
Abstract
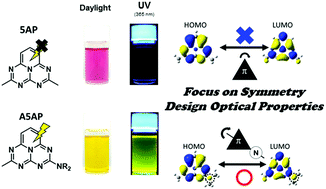
This communication describes the transformation of a non-emissive heterocycle into a luminophore via modulation of molecular orbitals by employing a dialkylamine-substituted pentaazaphenalene (A5AP) skeleton. It was presumed that the introduction of the amine group changed the symmetry-forbidden HOMO–LUMO (H–L) transition to an allowed one. According to optical measurements and theoretical calculations, the H–L transition was turned into the symmetry-allowed one because of the lone pair on the nitrogen atom in the dialkylamine substituent. Finally, the A5AP derivatives presented significant emission from the H–L transition.


 Please wait while we load your content...
Please wait while we load your content...