Stereospecific functionalizations and transformations of secondary and tertiary boronic esters
Abstract
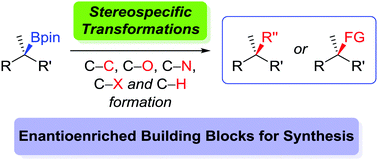
The formation of highly enantioenriched boronic esters through both stoichiometric and catalytic methods has received much attention over the past decade. Accordingly, the transformations of the boronic ester moiety into other functional groups is of considerable interest in synthesis. Specifically, transformations which retain the high enantioenrichment of the starting boronic ester, either through a stereoretentive or a stereoinvertive pathway, lead to the formation of new C–C, C–O, C–N, C–X, or C–H bonds at stereogenic centres. This feature article summarises the current state of the art in stereospecific transformations of both secondary and tertiary boronic esters into other functionalities and groups, whilst considering critically the transformations that are currently unattainable and would represent future advances to the field.
- This article is part of the themed collections: Celebrating our 2025 Prizewinners, ChemComm 60th Anniversary Historic Papers from the United Kingdom, Most cited articles from 2017, Most cited Features from 2016, 2017, Most downloaded articles of 2017: Organic and Biological Chemistry and Celebrating the 2017 RSC Prize and Award Winners


 Please wait while we load your content...
Please wait while we load your content...