Regioselective SN2 reactions for rapid syntheses of azido-inositols by one-pot sequence-specific nucleophilysis†
Abstract
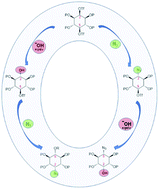
Triflates of myo-inositol undergo facile solvolysis in DMSO and DMF yielding SN2 products substituted with O-nucleophiles; DMF showed slower kinetics. Axial O-triflate undergoes faster substitution than equatorial O-triflate. By exploiting this difference in kinetics, solvent-tuning and sequence-controlled nucleophilysis, rapid synthesis of three azido-inositols of myo-configuration from myo-inositol itself has been achieved.
- This article is part of the themed collection: Site-selective molecular transformations


 Please wait while we load your content...
Please wait while we load your content...