Ring-opening of cyclic ethers by aluminum hydridotriphenylborate†
Abstract
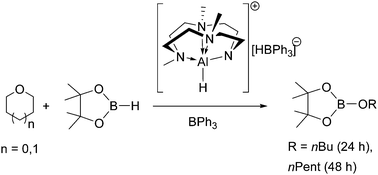
Molecular aluminum hydride [(L)AlH2] (L = Me3TACD) reacted with 2 equiv. of BPh3 in THF or THP to give the cationic alkoxides [(L)Al(OR)][HBPh3] (R = nBu, nPent) by facile ring-opening of the cyclic ethers. The Cα–O bond cleavage which involves the isolable intermediate [(L)AlH][HBPh3] is a result of hydride transfer to Cα from [HBPh3]−.


 Please wait while we load your content...
Please wait while we load your content...