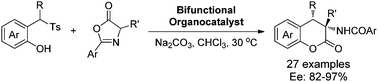
Bifunctional squaramide-catalyzed synthesis of chiral dihydrocoumarins via ortho-quinone methides generated from 2-(1-tosylalkyl)phenols†
Abstract
A bifunctional squaramide-catalyzed reaction of azlactones with o-quinone methides in situ generated from 2-(1-tosylalkyl)-phenols has been successfully developed under basic conditions, providing an efficient and mild access to chiral dihydrocoumarins bearing adjacent tertiary and quaternary stereogenic centers in high yields with excellent diastereo- and enantioselectivities.


 Please wait while we load your content...
Please wait while we load your content...