Intracellular synthesis of d-aminoluciferin for bioluminescence generation†
Abstract
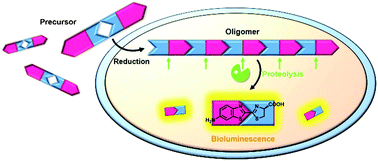
D-Luciferin is the most widely used substrate for bioluminescence (BL) applications but its low chemical stability always affects its performance. Herein, we rationally designed two chemically stable precursor molecules CBT-D-cystine-CBT (D-1) and CBT-L-cystine-CBT (L-1), and subjected them to reduction-controlled condensation to form 1-oligomer and subsequent proteolysis to yield D-aminoluciferin for BL generation in cells and in vivo. We envision that our precursor molecules might serve as D-luciferin alternatives for a wide range of BL applications in the near future.


 Please wait while we load your content...
Please wait while we load your content...