Exhaustive Suzuki–Miyaura reactions of polyhalogenated heteroarenes with alkyl boronic pinacol esters†
Abstract
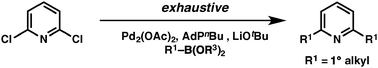
A novel Suzuki–Miyaura protocol is described that enables the exhaustive alkylation of polychlorinated pyridines. This method facilitates a formal synthesis of normuscopyridine and the rapid assembly of a dumbbell shaped portion of a [2]rotaxane.
- This article is part of the themed collection: 2017 Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...