Reversible crystal-to-crystal chiral resolution: making/breaking non-bonding S⋯O interactions†
Abstract
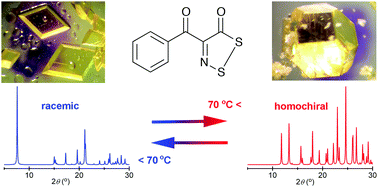
An achiral organic molecule adopts a chiral conformation, crystallizing in two morphologies: a racemic form, stable <70 °C, and a homochiral form, stable ≥70 °C. Upon seeding, crystal-to-crystal phase transitions occur reversibly between the racemic and chiral forms. Liquid-to-solid chiral crystallization is observed >90% of the time from the melt.


 Please wait while we load your content...
Please wait while we load your content...