Palladium-catalyzed intermolecular amination of unactivated C(sp3)–H bonds via a cleavable directing group†
Abstract
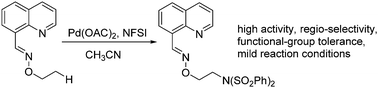
Palladium-catalyzed intermolecular amination of unactivated C(sp3)–H bonds was developed. Using NFSI as both the amino source and the oxidant, this protocol operates under mild conditions with excellent terminal selectivity and a broad substrate scope. Moreover, the directing group can be easily removed to produce 1,2-amino alcohols.


 Please wait while we load your content...
Please wait while we load your content...