Iridium(iii) catalyzed regioselective amidation of indoles at the C4-position using weak coordinating groups†
Abstract
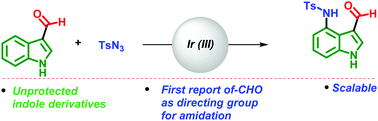
The C4-aminated indole scaffold is frequently encountered in several natural products and biologically active compounds. Herein we disclose a simple and short synthetic route for the amidation of indoles at the C4 position by employing an aldehyde as a directing group and Ir(III) as a catalyst. This strategy offers high selectivity for the C4-amidation of unprotected and protected indoles. A simple deprotection of the tosyl group leads to the formation of C4-amino indole derivatives, which are useful synthons for synthesizing natural products in the teleocidin family.
- This article is part of the themed collection: Most cited articles from 2017


 Please wait while we load your content...
Please wait while we load your content...