Electrodeposited-hydroxide surface-covered porous nickel–cobalt alloy electrodes for efficient oxygen evolution reaction†
Abstract
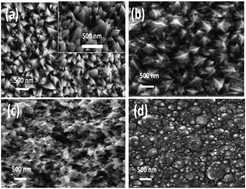
Herein, we present the electrochemical fabrication of a hydroxide surface-covered nickel–cobalt alloy and its superior catalytic activity towards the electrochemical oxygen evolution reaction in alkaline media. Electrodes were fabricated via the galvanostatic and potentiostatic electrodeposition methods in various electrolytic compositions, in which a nanopyramid-like morphology was obtained under the galvanostatic conditions and a porous structure was obtained under the potentiostatic deposition conditions. The optimized porous electrode shows the overpotential of 1.537 V (η = 307 mV) vs. RHE for the current density of 10 mA cm−2 in 1 M NaOH solution. Comparatively, the porous Ni–Co alloy electrode exhibits a better performance than the other morphologies obtained in this study. Owing to its highly active surface area with porous structures, it promotes the molecular oxygen evolution reaction.


 Please wait while we load your content...
Please wait while we load your content...