A cobalt(ii) complex with unique paraSHIFT responses to anions†
Abstract
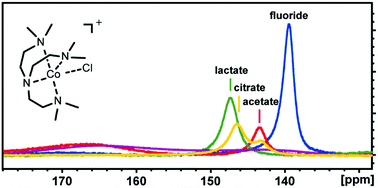
ParaSHIFT agents have shown promise in detecting chemical targets in biological systems by magnetic resonance, but few studies have used transition metal complexes for this purpose. Here we report our investigations into CoMe6trenCl (tren = tris(2-aminoethyl)amine) as a paraSHIFT agent. The paramagnetic region of the 1H NMR spectrum shows characteristic spectral profiles in the presence of fluoride, acetate, lactate and citrate in aqueous solution. These distinctive NMR shifts of each anion are maintained even in mixtures of anions.
- This article is part of the themed collection: Celebrating Excellence in Research: 100 Women of Chemistry


 Please wait while we load your content...
Please wait while we load your content...