Construction of the septahydroxylated ABC-ring system of dihydro-β-agarofurans: application of 6-exo-dig radical cyclization†
Abstract
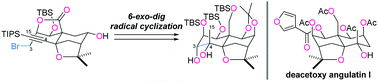
A synthetic route to the septahydroxylated ABC-ring system of dihydro-β-agarofurans was established. The B-ring was formed by a base-promoted diastereoselective Diels–Alder reaction between 3-hydroxy-2-pyrone and a D-glyceraldehyde-derived dienophile, while the C-ring was cyclized by PhSeCl-mediated etherification. The remaining A-ring was constructed via a 6-exo-dig radical reaction. Selective transformations gave rise to the ABC-ring system 1 with nine contiguous stereocenters. The thus obtained 1 corresponded to the enantiomer of the densely oxygenated core structure of dihydro-β-agarofurans.


 Please wait while we load your content...
Please wait while we load your content...