Total synthesis of nannocystin Ax†
Abstract
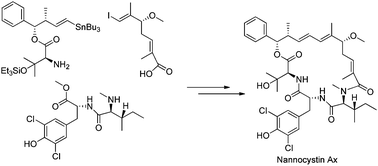
A concise total synthesis of nannocystin Ax, a natural depsipeptide recently isolated from myxobacteria, has been accomplished. By following a convergent strategy, the target molecule was assembled from three fragments. Each fragment can be synthesized expeditiously from readily achievable compounds. The key elements in this total synthesis feature Kobayashi's remote asymmetric induction with vinylketene silyl N,O-acetal, Roush's asymmetric crotylboration of aldehyde, Mitsunobu's esterification and macrocyclization via Stille cross-coupling.


 Please wait while we load your content...
Please wait while we load your content...