A catalytic enantioselective approach to tetrol bearing vicinal all-carbon quaternary stereogenic centers†
Abstract
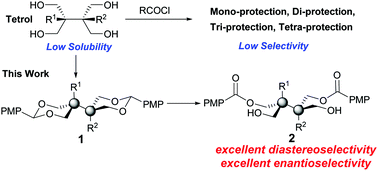
The first highly enantioselective catalytic protocol for selective manipulation of tetrol benzylidene acetals through chiral phosphoric acid mediated oxidative desymmetrization is reported. This efficient approach provides a general access to vicinal all-carbon quaternary stereogenic center structural motifs, as well as structures containing vicinal tertiary and quaternary stereocenters enantioselectively. The synthetic utility has been furthermore demonstrated by transformations and gram-scale processes.


 Please wait while we load your content...
Please wait while we load your content...