Metal-free benzannulation of 1,7-diynes towards unexpected 1-aroyl-2-naphthaldehydes and their application in fused aza-heterocyclic synthesis†
Abstract
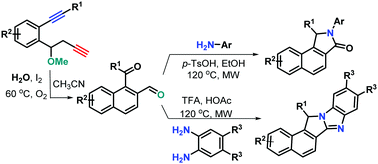
A novel I2-mediated benzannulation of 1,7-diyne-involved 1,4-oxo-migration was established, providing a range of unexpected 1-aroyl-2-naphthaldehydes with a 1,4-dicarbonyl unit. The resulting 1-aroyl-2-naphthaldehydes were successfully applied in the synthesis of benzo[e]isoindol-3-ones and benzo[e]benzo[4,5]imidazo[2,1-a]isoindoles using aromatic amines and benzene-1,2-diamines as nucleophiles, respectively. The mechanisms for the formation of these compounds were proposed.


 Please wait while we load your content...
Please wait while we load your content...