Aromatic metamorphosis: conversion of an aromatic skeleton into a different ring system
Abstract
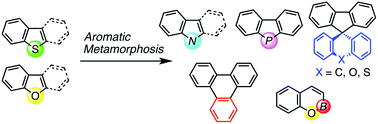
Aromatic skeletons are generally regarded as being uncleavable because of their aromatic stabilization energy. Compared to exocyclic functionalizations of aromatic compounds, much less attention has been paid to substitutions of endocyclic atoms in aromatic cores through partial disassembly of the cyclic skeletons and subsequent ring reconstruction. In this Feature Article, we describe our endeavours to establish “aromatic metamorphosis”, where general aromatic compounds such as dibenzothiophenes, dibenzofurans, and benzofurans are transformed into different ring systems using a multi-step strategy or ideally in one step.


 Please wait while we load your content...
Please wait while we load your content...