Transition-metal-free, visible-light induced cyclization of arylsulfonyl chlorides with o-azidoarylalkynes: a regiospecific route to unsymmetrical 2,3-disubstituted indoles†
Abstract
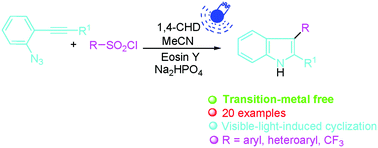
A visible-light-catalyzed synthesis of unsymmetrical 2,3-diaryl-substituted indoles from arylsulfonyl chlorides and o-azidoarylalkynes at room temperature has been discovered. This transformation exhibits excellent substrate scope and functional group tolerance. The use of inexpensive eosin Y as the catalyst with easy operation makes this protocol very practical.


 Please wait while we load your content...
Please wait while we load your content...