Ni-Catalyzed regio- and stereoselective addition of arylboronic acids to terminal alkynes with a directing group tether†
Abstract
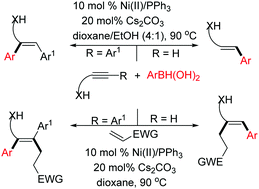
Addition of arylboronic acids to directing group tethered acetylenes in a regio and stereoselective manner using an inexpensive catalytic system is achieved for the first time to access highly sought after allyl/homoallyl alcohol/amine units. The apparent vinylnickel intermediate was successfully trapped by the Michael electrophiles to get defined tri- and tetra-substituted olefins. An interesting selectivity switch was observed with internal alkynes.


 Please wait while we load your content...
Please wait while we load your content...