Accessing alternative reaction pathways of the intermolecular condensation between homo-propargyl alcohols and terminal alkynes through divergent gold catalysis†
Abstract
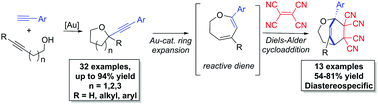
An intermolecular condensation of alkynols and terminal alkynes is reported. Using IPrAuNTf2, an efficient Au-catalyzed cyclization–alkynylation strategy furnishes (2-arylalkynyl) cyclic ethers in moderate to excellent yields (up to 94%). This strategy is extended to the synthesis of functionalized 2,3-dihydrooxepines via the sequential Au-catalyzed ring expansion of the cyclic ether substrates.

 Please wait while we load your content...
Please wait while we load your content...