The biodistribution, excretion and potential toxicity of different-sized Pd nanosheets in mice following oral and intraperitoneal administration†
Abstract
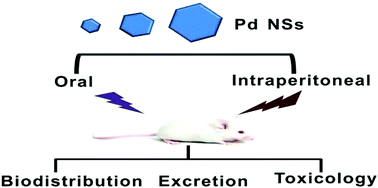
Two-dimensional (2D) Pd-based nanomaterials with strong near-infrared absorption have recently shown great application prospects in cancer diagnosis and therapy. Most previous studies mainly focused on understanding the in vivo behaviors and treatment effects of these Pd-based nanomaterials after intravenous injection into mice. However, it remains unclear whether other administration routes will affect the in vivo biodistribution, excretion and potential toxicity of Pd-based nanomaterials. In this study, for the first time we systematically explored the in vivo behaviors of different-sized Pd nanosheets (NSs) (approximately 5 nm, 30 nm and 80 nm) following oral feeding and intraperitoneal injection. It was found that Pd NSs with oral administration had a rather low accumulation that decreased with time in all examined organs, and became almost undetectable in these organs at 24 h post-injection. In comparison, the intraperitoneally injected Pd NSs exhibited obvious time-dependent and size-dependent accumulations in the reticuloendothelial (RES) system including the liver and spleen within 24 h post-injection, and then the accumulation amounts decreased with the lapse of time. Moreover in tumor tissue, smaller-sized Pd NSs (5 nm) had a higher uptake than larger-sized Pd NSs (30 nm and 80 nm). Excretion studies uncovered that more than 70% ID of Pd NSs could be rapidly excreted from the body through urine and feces within two days after oral administration, whereas Pd NSs with intraperitoneal injection could be gradually cleared, mainly via urine within 8 days. Further histological examination of organ sections and blood biochemical analysis evidenced that these different-sized Pd NSs do not cause obvious toxicity in the treated mice at the tested period with the given dose. These results not only indicate that the biodistribution and excretion capabilities of Pd NSs are closely related to their administration routes, but also imply that the intraperitoneally injected Pd NSs have greater potential for in vivo biomedical studies compared to oral feeding, because of their relatively higher tissue absorption and gradual excretion from the body. This study will provide valuable information for the clinical translation of 2D Pd-based nanomaterials.

 Please wait while we load your content...
Please wait while we load your content...