Anti-inflammatory effects of octadecylamine-functionalized nanodiamond on primary human macrophages†
Abstract
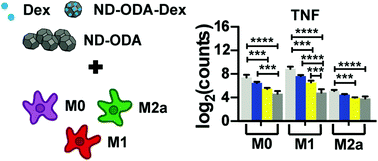
Chronic inflammatory disorders such as rheumatoid arthritis are characterized by excessive pro-inflammatory or “M1” activation of macrophages, the primary cells of the innate immune system. Current treatments include delivery of glucocorticoids (e.g. dexamethasone – Dex), which reduce pro-inflammatory M1 behaviour in macrophages. However, these treatments have many off-target effects on cells other than macrophages, resulting in broad immunosuppression. To limit such side effects, drug-incorporated nano- and microparticles may be used to selectively target macrophages via phagocytosis, because of their roles as highly effective phagocytes in the body. In this study, surface-modified nanodiamond (ND) was explored as a platform for the delivery of dexamethasone to macrophages because of ND's rich surface chemistry, which contributes to ND's high potential as a versatile drug delivery platform. After finding that octadecylamine-functionalized nanodiamond (ND-ODA) enhanced adsorption of Dex compared to carboxylated ND, the effects of Dex, ND-ODA, and Dex-adsorbed ND-ODA on primary human macrophage gene expression were characterized. Surprisingly, even in the absence of Dex, ND-ODA had strong anti-inflammatory effects, as determined by multiplex gene expression via NanoString and by protein secretion analysis via ELISA. ND-ODA also inhibited expression of M2a markers yet increased the expression of M2c markers and phagocytic receptors. Interestingly, the adsorption of Dex to ND-ODA further increased some anti-inflammatory effects, but abrogated the effect on phagocytic receptors, compared to its individual components. Overall, the ability of ND-ODA to promote anti-inflammatory and pro-phagocytic behaviour in macrophages, even in the absence of loaded drugs, suggests its potential for use as an anti-inflammatory therapeutic to directly target macrophages through phagocytosis.
- This article is part of the themed collection: Emerging Investigators 2017


 Please wait while we load your content...
Please wait while we load your content...