A selective nanosensor for ultrafast detection of Cu2+ ions based on C5 DNA-templated gold nanoclusters and Fenton-like reaction†
Abstract
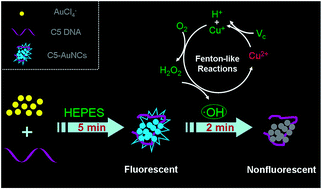
Copper pollution has become a very serious problem in modern society with the increasing industrial emission and the acid mine drainage. Copper deficiency or excess copper accumulation in human and animal livers can bring both oxidative stress and severe disorders. Thus, the development of a simple and efficient strategy for Cu2+ ion detection in water and blood samples is very necessary and meaningful. In this study, a simple, fast, and sensitive fluorescence method was put forward to detect Cu2+ ions based on C5 DNA-templated gold nanoclusters (AuNCs) and Fenton-like reactions. Under aerobic conditions, ascorbic acid is not only involved in the reduction of Cu2+, but also reacts with O2 to produce H2O2. Then, hydroxyl radical (˙OH), yielded by H2O2 and Cu+, can seriously quench the fluorescence of AuNCs. Attributed to the unique Fenton-like reaction between ascorbic acid and the Cu2+ ions, high selectivity was achieved for Cu2+ ion monitoring using this nanosensor, and the practical applications of this nanosensor in real water and blood samples were realized.


 Please wait while we load your content...
Please wait while we load your content...