Visual determination of ferric ions in aqueous solution based on a high selectivity and sensitivity ratiometric fluorescent nanosensor†
Abstract
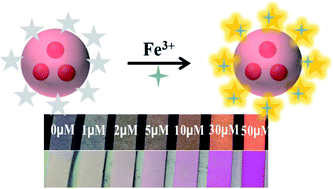
A novel ratiometric fluorescent nanosensor for visual sensing of Fe3+ has been developed by integrating yellow-emissive rhodamine derivatives (RhB) onto the surface of silica nanospheres embedded with red CdTe QDs. Such a nanohybrid fluorescent sensor, RhB–CdTe@SiO2 QDs, displays two distinct emission peaks 556 nm and 651 nm under a single excitation wavelength in the presence of Fe3+. The yellow fluorescence of RhB could be selectively increased by Fe3+ due to the strong chelating ability of RhB toward Fe3+, while the red fluorescence of CdTe QDs is almost constant, resulting in a distinct fluorescence color transformation from red to yellow, which can be applied for the visual detection of Fe3+. This approach shows high sensitivity and selectivity toward Fe3+, and the detection limit is determined to be 20.5 nM, which is lower than the maximum level (0.3 mg L−1, equivalent to 5.4 μM) of Fe3+ ions permitted in drinking water by the U.S. Environmental Protection Agency (EPA). For practical application, we dope the mixture of RhB–CdTe@SiO2 QDs and PVA onto the filter paper and fabricate test strips for direct visual inspection of Fe3+ in water. The paper based sensor has a visual detection limit of 1 μM by the naked eye, showing its promising application for onsite visual quantification of ferric ions without the need for elaborate equipment.


 Please wait while we load your content...
Please wait while we load your content...