Supercritical fluid (CO2) chromatography for quantitative determination of selected cancer therapeutic drugs in the prescence of potential impurities in injection formulations
Abstract
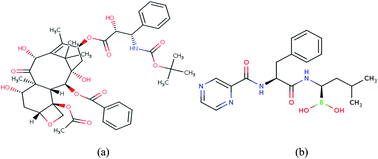
In the present study, two cancer therapeutic drugs (docetaxel and bortezomib) were separated from their potential impurities on a chromatographic platform by utilizing CO2 gas (supercritical state) and quantified. The chromatographic separations were achieved on two short columns BEH-2EP (100 mm × 3 mm, 1.7 μm) and CHIRALPAK AD-3 (100 mm × 4.6 mm, 3 μm) for docetaxel and bortezomib, respectively. The present work describes the role of organic modifiers in the separation of polar compounds by supercritical fluid chromatography. The two new methods were fully validated in accordance with the current ICH (International Council for Harmonization of technical requirements for pharmaceuticals for human use) guidelines. The stability indicating power of the methods was demonstrated from the stress studies conducted on the injection formulations of the two compounds. The methods are precise with % RSD of 0.4, linear with the correlation coefficient of r2 ≥ 0.999 and accurate in the range of 50–150% of the target assay concentration. The two methods can be equally employed for the assay determination of docetaxel and bortezomib APIs as well.


 Please wait while we load your content...
Please wait while we load your content...