All-solid-state ion-selective electrodes with redox-active lithium, sodium, and potassium insertion materials as the inner solid-contact layer†
Abstract
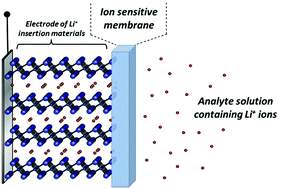
All-solid-state ion-selective electrodes as potentiometric ion sensors for lithium, sodium, and potassium have been demonstrated by installing a composite layer containing a powder of alkali insertion materials, LixFePO4, Na0.33MnO2, and KxMnO2·nH2O, respectively, as an inner solid-contact layer between the electrode substrate and plasticized poly(vinyl chloride) (PVC)-based ion-sensitive membrane containing the corresponding ionophores for Li+, Na+, and K+ ions. These double-layer ion-selective electrodes, consisting of the composite and PVC layers prepared by a simple drop cast method, exhibit a quick potential response (less than 5 s) to each alkali–metal ion with sufficient Nernstian slopes of calibration curves, ca. 59 mV per decade. The installation of the insertion materials as the inner solid-contact layers is highly efficient for the stabilization of membrane potential, resulting in a prompt response to the alkali ion activity in the analyte, compared to those of the electrodes without the alkali insertion materials. From alternating current impedance measurements for the electrodes, the inner layer of the installed alkali insertion materials drastically reduces the impedance of the membrane/electrode interface, leading to an improvement in their ion-sensing performance.


 Please wait while we load your content...
Please wait while we load your content...