C–H oxidation and chelation of a dipyrromethane mediated rapid colorimetric naked-eye Cu(ii) chemosensor†
Abstract
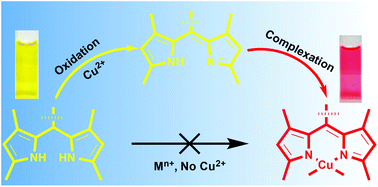
Copper(II) ion mediated C–H oxidation of dipyrromethanes (DPMs) to the corresponding dipyrrins followed by complexation invoked the selective sensing of copper(II) ions in aqueous solutions. On the addition of copper, the colour of the DPM solution instantaneously changes from yellow to pink with the detection limit of 0.104 μM measured by absorption spectroscopy, whereas visible colour changes could be observed by the naked eye for concentrations as low as 3 μM.


 Please wait while we load your content...
Please wait while we load your content...