Highly efficient enrichment of N-linked glycopeptides using a hydrophilic covalent-organic framework†
Abstract
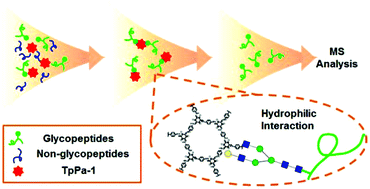
The enrichment of glycopeptides plays an important role in glycoproteomics. In this paper, a covalent-organic framework called TpPa-1, synthesized by the Schiff base reaction of 1,3,5-triformylphloroglucinol and paraphenylenediamine, was first successfully utilized as a hydrophilic porous material for N-linked glycopeptide enrichment. Using this material, interference from non-glycopeptides could be efficiently eliminated, which facilitated the mass spectrometry detection of glycopeptides. By capturing N-linked glycopeptides from tryptic digests of human IgG, our method was proved to have high sensitivity at the femtomole level. And it showed superior selectivity for glycopeptides even when non-glycopeptides were 1000 times more concentrated. Due to the strong covalent bonds, this material possessed good stability and could be repeatedly used for at least 10 times. The ultra-low mass density and abundant binding sites also provided it with high binding capacity (178 mg g−1, IgG/TpPa-1). Moreover, N-linked glycopeptides were easily enriched by this material from only 10 μL human serum, which demonstrated its potential in pretreatment of complex biological samples.


 Please wait while we load your content...
Please wait while we load your content...