Fluorescence recognition of chiral amino alcohols by using a novel ionic liquid sensor†
Abstract
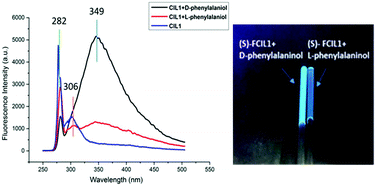
A novel task-specific ionic liquid derived from L-phenylalaninol was prepared as an enantioselective fluorescent sensor for the first time. Fluorescent chiral ionic liquid 1 (FCIL1) is found to exhibit highly enantioselective fluorescence enhancements toward both aromatic and non-aromatic chiral amino alcohols. When (S)-FCIL1 was treated with the enantiomers of phenylalaninol, a great fluorescence enhancement at 349 nm could be observed and the value of the enantiomeric fluorescence difference (ef) is 5.92. This demonstrated that the chiral sensor (S)-FCIL1 exhibited an excellent enantioselective response behaviour to D-phenylalaninol. Besides that, both the fluorescence intensity at 349 nm (I349) and the ratio of I349 to I282 depend linearly on the concentration of amino alcohols. Both the concentration and the enantiomeric composition could be determined by using the chiral ionic liquid. Differently, the sensor treated with the enantiomers of 2-amino-1-butanol showed an opposite result: the fluorescence intensity of the S-enantiomer is higher than that of the R-enantiomer. Furthermore, the size of the substituents on the chiral carbon might be important for the enantioselective fluorescent response.


 Please wait while we load your content...
Please wait while we load your content...