Chemoselective ligation reaction of N-acetylglucosamine (NAG) with hydrazide functional probes to determine galactosyltransferase activity by MALDI mass spectrometry†
Abstract
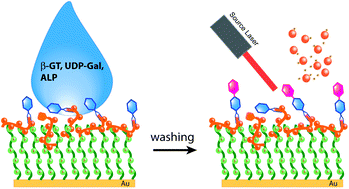
Quantification of β-1,4-galactosyltransferase (β-1,4-GT) activity is of considerable significance in the diagnosis of various cancers including lung and ovarian cancer. We report here the use of synthetic β-N-acetylglucosamine (NAG) ligands that contain hydrazide functional groups to determine galactosyltransferase activity by mass spectrometry. With hydrazide-linked β-D-NAG as the acceptor, the activity of β-1,4-GT is quantified by matrix-assisted laser desorption/ionization (MALDI)-time-of-flight (TOF) mass spectrometry (MS) with high efficiency. Using the disulfide moiety in a 3,3′-dithiodipropionic acid dihydrazide (DTP)-linked β-D-NAG probe, Au nanoparticles (AuNPs) are employed for enriching DTP-linked β-D-NAG after enzymatic reaction, and the ligand-bound AuNPs are subsequently deposited on a MALDI plate for analysis. In addition, we have demonstrated that a perfluorocarbon (PF) labeled β-NAG-ligand can be useful for surface-based enzymatic reaction with a perfluorooctadecanethiol (PFDT)-covered gold surface. Using the ratiometric method, the conversion rate of β-1,4-GT is determined to be 0.83 ± 0.03, which shows a high level of activity. This is the first work that uses hydrazide-linked β-D-NAG for activity analysis, providing a new surface-based MS approach to determine enzyme activity in a potentially high-throughput manner.


 Please wait while we load your content...
Please wait while we load your content...